Related Research
The PaceLab laboratory investigates the properties of cardiac ion channels. Our main project concerns the pacemaker ("funny") If current, first described in the pacemaker cells of the sinoatrial node (SAN) (Brown, DiFrancesco & Noble, 1979) and later characterized in cardiac myocytes and neurons.
Cardiac rhythmic activity is generated by "pacemaker" cells, which in mammals are located in the SAN. Action potentials of SAN cells have a special phase, called diastolic (or pacemaker) depolarization. At the end of an action potential, the pacemaker depolarization slowly moves the membrane voltage up to threshold for firing of a new action potential, thus generating repetitive activity. The mechanism responsible for initiation of the process of pacemaker depolarization is the activation during diastole of the pacemaker “funny” (If) current. As well as by voltage hyperpolarization, If is activated by intracellular cAMP (DiFrancesco & Tortora, 1991), and is therefore modulated by the autonomic neurotransmitters noradrenaline (which activates If) by increasing intracellular cAMP, thus accelerating rate) and acetylcholine (which inhibits If) by the decreasing intracellular cAMP, thus slowing rate). These mechanisms are therefore responsible for the modulation of cardiac rate by the autonomic nervous system (DiFrancesco Ducouret & Robinson, 1989; DiFrancesco, 1993).An If -like current (Ih) has also been described in a wide variety of neuronal cells, both in the central and peripheral nervous system, where it has different, extremely important functional roles (Robinson & Siegelbaum, 2003). In sensory neurons, Ih is involved in the perception of external stimuli, or in modulating the transduction of sensory stimuli into electrical signalling. Ih is also expressed in pre-synaptic membranes, where it is involved in plasticity phenomena.
Substantial advancement in the knowledge of the molecular properties of pacemaker channels was achieved with the cloning in the late ´90s of the HCN family of channels (Hyperpolarization-activated, Cyclic-Nucleotide-gated). HCN channels belong to the super-family of K+ voltage-dependent (Kv) and cyclic-nucleotide gated (CNG) channels and are the molecular determinants of native pacemaker channels in the heart and nervous system. Of the 4 isoforms cloned (HCN1-4), HCN4 is the most highly expressed in cardiac pacemaker tissue, though also HCN1 and HCN2 are expressed to a lesser extent in atrial and ventricular tissues. HCN1, HCN2 and HCN4 isoforms are also highly expressed in central and peripheral neurons (Biel et al 2009).
As well as representing a fundamental mechanism in the physiology of the generation and control of cardiac rhythmic activity, the concept funny channel-based pacemaking can be exploited in clinically relevant applications. As highlighted in DiFrancesco, 2020:
“Present knowledge of the basic mechanisms of pacemaking and the properties of funny channels has led to important developments of clinical relevance. These include: 1) the successful development of heart rate-reducing agents, such as ivabradine, able to control cardiac rhythm and useful in the treatment of diseases such as coronary artery disease, heart failure and tachyarrhythmias; 2) the understanding of the genetic basis of disorders of cardiac rhythm caused by HCN channelopathies; 3) the design of strategies to implement biological pacemakers based on transfer of HCN channels or of stem cell-derived pacemaker cells expressing If, with the ultimate goal to replace electronic devices.”
Our laboratory applies the most advanced techniques of electrophysiology, biochemistry, immunofluorescence, molecular and cellular biology and other techniques, to investigate basic and complex features of HCN channels and potential translational applications.
Funny current
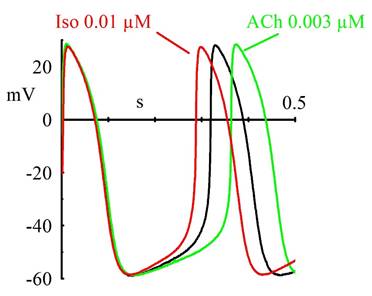
Cardiac rhythmic activity is generated by "pacemaker" cells, which in mammals are located in the sinoatrial node (SAN). Action potentials of SAN cells have a special phase, called diastolic (or pacemaker) depolarization (DD): at the end of an action potential, the pacemaker depolarization slowly takes the membrane voltage up to threshold for firing of a new action potential, thus generating repetitive activity. What is the mechanism underlying the pacemaker depolarization?
In 1979, Brown, DiFrancesco & Noble described a "funny" (If) current, so called because of its unusual properties, in cardiac pacemaker cells. Since If was inward and slowly activating on hyperpolarization at voltages in the diastolic range, the authors proposed that it was involved in the generation of spontaneous activity; the current was also increased by adrenaline, indicating an involvement in the catecholamine-induced acceleration of pacemaker activity and hence of heart rate. In another cardiac preparation displaying spontaneous activity, the Purkinje fibre, a "pacemaker" current had been observed previously, but it had been described by as a pure K+ current, outward at diastolic voltages (IK2 current, Noble & Tsien, 1968). The Purkinje fibre's IK2 current was similar to the nodal If in several aspects, and the apparently different ionic nature of the two currents remained a puzzling issue until in 1981, a reinterpretation of the IK2 current was proposed (DiFrancesco 1981a,b) demonstrating that the two currents were indeed identical, and IK2 had been erroneously interpreted for over 10 years as a pure K+ current. This demonstrated the identity of pacemaker components in different cardiac cells. Following its early description, If was extensively investigated in cardiac cells (DiFrancesco, 1993). These studies showed that If is a mixed Na+ and K+ current, activated on hyperpolarization from a threshold of about -40/-50 mV. When at the end of an action potential the membrane repolarizes below this threshold, If is activated and supplies inward current flow, which is responsible for generation of the diastolic depolarization phase. If is also modulated by intracellular cAMP, by an action exerted directly on f-channels and not mediated by phosphorylation, as first shown by DiFrancesco and Tortora, 1991. Raising cAMP shifts the If activation curve to more positive voltages, thus providing more inward current for the pacemaker depolarization. This action mediates the modulation of cardiac rate by the autonomic nervous system. In cardiac cells, cAMP synthesis is stimulated and inhibited by ß-agonists and muscarinic agonists, respectively; thus, sympathetic stimulation accelerates, and vagal stimulation slows, cardiac rate by increasing and decreasing, respectively, If at diastolic potentials via changes in cAMP level. Importantly, moderate vagal activity, such as the one associated to basal vagal tone, controls cardiac rate by modulation of If, rather than by the opening of ACh-activated K+ channels (current IK,ACh), as was previously thought (DiFrancesco, Ducouret & Robinson, 1989).
If has a very low single-channel conductance, in fact the lowest ever recorded by single-channel patch recording (about 1 pS, DiFrancesco, 1986). Binding of cAMP molecules to f-channels increases the probability of channel opening upon hyperpolarization (DiFrancesco & Mangoni, 1994). This mechanism can be interpreted in terms of an allosteric cAMP-induced channel activation whereby cAMP binds preferentially, and thus stabilizes, the "open" configuration of the channel (DiFrancesco, 1999).
The funny current has been described also in a variety of neuronal preparations, where it is dubbed Ih current. The role of Ih in neurons is based on its ability to generate a depolarization following either membrane hyperpolarization, or a receptor- mediated modification of intracellular cAMP. Thus, Ih can contribute to control of resting potential and modulation of excitability, as well as to firing discharge. Dedicated review literature highlights the many functional roles of f/h channels in health and disease in neuronal as well as in cardiac cells (Robinson & Siegelbaum, 2003; DiFrancesco & DiFrancesco, 2015; Rivolta et al 2020; DiFrancesco et al 2019; Combe & Gasparini, 2021; Porro et al 2021).
Genetics of pacemaker channel diseases
Cardiac diseases associated with rhythm disorders are among the major causes of morbidity and mortality in western countries. Rhythm disorders can be caused typically by genetic mutations of ion channels involved in cardiomyocyte’s electrical activity.
Given the role of the If current in pacemaker activity, it is no surprise that dysfunctional mutations of HCN4 channels (the molecular components of native funny channels) can lead to arrhythmias. Indeed, several HCN4 mutations have been identified in patients with alterations of cardiac rhythm (DiFrancesco 2013). Identified arrhythmias can be complex and include tachy-brady syndrome, AV-block, atrial fibrillation, chronotropic incompetence and others, but the majority of reported mutations are loss-of-function (i.e., leading to reduced channel contribution to pacemaker activity) and are associated with bradycardia, in agreement with the funny channel role in rate control.
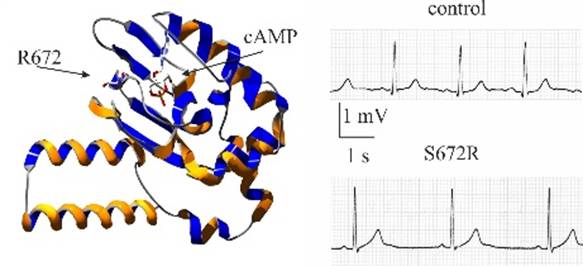
In one of the earliest such reports presenting the first family-wide study of HCN-linked rhythm disorders, the PaceLab identified a mutation (S672R) in the hHCN4 gene responsible for a familial form of asymptomatic bradycardia (Milanesi et al., 2006). Heterologous expression and patch-clamp analysis showed that “mutant channels are activated at more negative voltages than are wild-type channels. These changes, which mimic those of mild vagal stimulation, slow the heart rate by decreasing the inward diastolic current”. This result was the first to provide functional demonstration that “diminished function of pacemaker channels is linked to familial bradycardia” (Milanesi et al., 2006).
While most frequent HCN4 mutations are loss-of-function and cause bradycardia, it is interesting to note that the only one tachyarrhythmia-associated mutation reported so far (Baruscotti et al 2015) is gain-of-function, which fully explains rhythm acceleration. HCN4 mutation-linked arrhythmias thus support the pacemaking role of If.
Further studies will be necessary to address how knowledge of the properties of human HCN4 channels can be exploited to limit harmful effects of HCN4-mutation-induced rhythm alterations and rescue normal pacing.
Pharmacology of the pacemaker channel

Drugs able to selectively block funny channels have a potential therapeutic use whenever a specific heart rate reduction, devoid of side effects, is desirable. Indeed, pharmacological research has been long seeking for substances able to slow heart rate specifically, and several “heart rate-inhibiting” substances have been developed to this aim.
Ivabradine is the only such drug having reached the market and is now marketed in many countries with the commercial name of Procoralan or Corlentor (or other names). Ivabradine is a blocker of funny channels and slows heart rate with little or no side effects. The drug is successfully used in the therapy of stable angina, ischaemic heart disease and heart failure (DiFrancesco & Camm 2004; DiFrancesco & Borer 2007), and its efficacy in off-label use to treat specific supraventricular arrhythmias such as Inappropriate Sinus Tachycardia (IST) has also been investigated (Cappato et al 2012).
The heart-rate inhibiting action of ivabradine is due to a selective block of funny channels. Early work by the PaceLab has shown that ivabradine blocks sinoatrial If in a use- and current-dependent way (Bucchi et al., 2002). Use-dependence means that the effect of drug application augments during repetitive activity, a feature resulting from accumulation of If current inhibition during repetitive activation/deactivation protocols (see for example figure 1 of Bucchi et al (2002)). This is therapeutically useful since it implies that the slowing action of ivabradine is stronger at higher heart rates (tachycardia), when the slowing action is most valuable.
Extending the characterization of the blocking effect of ivabradine to the HCN isoforms HCN1 and HCN4 has revealed unexpected differences in the mechanism of block of the two isoforms: ivabradine is an "open channel" blocker of native funny and HCN4 channels, but a "closed channel" blocker of the HCN1 isoform (Bucchi et al, 2006; DiFrancesco 2006). Differences in the modes of action of the drug can be exploited to develop new molecules with higher isoform-specificity.
Combining mutagenesis investigation and homology modelling of the HCN4 model has then provided the first evidence that ivabradine binds to the channel within an inner channel cavity below the pore, and interacts with specific residues lining the same cavity. That study provided an interpretation of previously identified properties of f/HCN4 channel block by ivabradine including the ‘‘open channel block’’, the current-dependence of block and the property of "trapping" of drug molecules in the closed configuration (Bucchi et al 2013). Docking of ivabradine molecules to the inner cavity of a Cryo-EM-resolved structure of the HCN4 channel has been confirmed more recently (Saponaro et al, 2021, figure 5).
More detailed knowledge of structural molecule-channel interaction will be useful in the perspective of the development of isoform-specific blockers. This is especially desirable in view of the role of defective HCN1 and HCN2 isoforms in neurological diseases such as epilepsy and neuropathic/inflammatory pain transmission.
Biological Pacemaker
Cardiac arrhythmias include complex and multifactorial disorders of cardiac rhythm. In the Sinus Node Disease (SND) syndrome, the sinoatrial node (SAN), the natural cardiac pacemaker region of the heart, fails to generate or propagate normal action potentials. This may lead to severe bradycardia and atrio-ventricular node block, conditions that often require implantation of an electronic pacemaker as the only possible therapy.
The aim of “biological pacemaker” studies is to create, within otherwise silent or defective tissue, a cellular substrate able to autonomously generate rhythmic, spontaneous activity, with the ultimate goal of eventually replacing the electronic devices implanted today with biological ones.
Various gene-therapy or cell-therapy approaches have been investigated to this aim. Among these, several attempts have shown that in situ delivery of funny/HCN channels to silent or defective cardiac muscle by gene- or cell-based methods can be successfully employed. The underlying idea is that along with f-channels, also their “pacemaking” function is transfected, so that silent cells made to express f-channels become spontaneously active.
Excellent review work covers this subject (see for example Rosen et al. 2011). Early studies showed the viability of various HCN-delivery protocols using for example adenoviral-mediated HCN infection, HCN channel-expressing mesenchymal stem cells, fusion between HCN1-expressing fibroblasts and myocytes. More recent attempts were based on the transfer of f-channel-expressing cardiomyocytes derived from embryonic stem cells. This approach is based on an in vitro differentiation of stem cells (embryonic or adult) towards a cardiac, pacemaker-like phenotype during which cells are driven to acquire a set of ion channels and other proteins necessary to generate spontaneous action potentials. The functional validation of the system includes: a) characterization of electrical properties of stem cell-derived cardiomyocytes (voltage- and current-clamp); b) analysis of ion channels and other sinoatrial proteins by RT-PCR, western blot, and confocal imaging; c) evaluation of the ability of these cells to modify and drive spontaneous activity of a primary ventricular cell culture. (Barbuti et al 2009; Scavone et al 2013). iPSC-derived pacemaker-like myocytes (Meraviglia et al 2016; Benzoni et al 2019) have also been used as a tool to transfer iPSC-derived pacemaker-like myocytes, and represent a promising tool to generate biological pacemakers, particularly since this approach can avoid immune rejection after transplant (Protze et al 2017).
Sinus Node Dysfunction (SND)
Also referred to as Sinus Node Disease or Sick Sinus Syndrome (SSS), the Sinus Node Dysfunction (SND) is a syndrome caused by the failure of the Sino Atrial Node (SAN), the natural pacemaker region of the heart, to generate and conduct a normal action potential. Most significant symptoms are persistent bradycardia, short/long episodes of sinus arrest, potentially associated with cardiac standstill or sinus exit block. Also typical of SND are tachy-brady syndromes, as well as “chronotropic incompetence”, defined as the inability of the heart to rapidly adjust to faster rates in the presence of increased physical activity or autonomic drive. Patients with symptomatic SND are subject to syncope, atrial fibrillation, heart failure and other adverse cardiovascular events, associated with an increased risk of cardiovascular death and overall mortality.
Symptomatic SND is the cardiac disease that most frequently requires pacemaker implantation and is responsible for some 50% of the total pacemaker implants. Given the heavy global burden of SND, innovative therapeutic strategies based on novel molecular insights into disease mechanisms would be highly beneficial.
SND is partly a structural disease, and its incidence increases in the ageing population in association with increased fibrosis of the SAN. Independently of ageing, SND is commonly associated with acquired heart disease (such as cardiac ischemia, atrial fibrillation, heart failure), inflammation, heart surgery, antiarrhythmic drug therapies etc. Idiopathic forms of the disease (that is, with no apparent cardiac abnormalities) are also known, and evidence for genetic causes has now been amply demonstrated.
Because of its role in the generation and control of spontaneous activity of the SAN, funny/HCN4 channels are obvious candidates in the search for genetic causes of SND. Indeed, several studies have shown the existence of specific HCN4 mutations leading to bradycardia and SND (DiFrancesco 2013). A platform for gaining novel insight into SND mechanisms has been proposed with FANTASY, a Transatlantic Network of Excellence project supported by the Leducq Foundation with the aim of “Fighting AgaiNsT sinus node dysfunction And aSsociated arrhYthmias” https://www.fondationleducq.org/network/fighting-against-sinus-node-dysfunction-and-associated-arrhythmias/.
The network concentrates the efforts of several European and US labs that together “present a research program focused on providing proof-of-concept knowledge for developing a new pharmacologic and molecular therapy for SND that aims to (i) normalize heart rate and rhythm in primary and secondary forms of SND and (ii) reverse the pathophysiological mechanisms leading to symptomatic SND”.
The PaceLab and Moroni labs of the Milano University contribute to the network by investigating mechanisms that regulate funny/HCN4 channel activity by three approaches:
1) in physiological conditions, by exploring cellular biochemical pathways involved in “long-term” channel modulation via phosphorylation and membrane trafficking (DiFrancesco 2019), as opposed to the “short-term” modulation mediated by cAMP, as originally described in 1991 (DiFrancesco & Tortora 1991);
2) in pathological conditions, by investigating in SND patients carrying mutated HCN4 channels, how HCN channel dysfunctional mutations affect generation, propagation and rate of spontaneous action potentials (Milanesi et al 2006; Baruscotti et al 2011; DiFrancesco 2020);
3) at the molecular level by the use of Cryo-Electron Microscopy, investigating mechanisms by which molecules directly interacting with HCN4 channels modify their activity (Saponaro et al 2021).
Information gained by these approaches will provide novel knowledge of SND-related HCN4 dysfunctional behaviour and allow development of new molecules interacting with HCN4 channels potentially useful for therapeutic interventions.
FANTASY TEAM

dario.difrancesco@unimi.it

luca.palloni@unimi.it

caterina.azzoni@studenti.unimi.it



